Table of Contents
- Consent Forms
Consent Forms
Updated
by Gina Romero
- Consent Forms
Consent Forms
Collect legally required participant agreements before they take part in any study — using built-in consent checkboxes or your own custom PDF consent forms.
What Are Consent Forms?
Consent forms ensure that participants are informed about your research and agree to your data usage terms before they participate. Great Question provides two consent options: Standard GQ consent checkboxes, which are enabled by default on every study, and Custom consent forms (only available on Enterprise plans), which let you upload your own PDF documents with your organization's specific legal language. Both options collect participant agreement before they can book, complete a survey, or start a task.
Why It Matters
Stay legally compliant. Ensure every participant agrees to your organization's research and data usage terms before they take part, meeting regional, company, or project-specific privacy requirements.
Reduce manual overhead. Signed consent forms are automatically stored in participant profiles, so you never have to chase down paperwork or track agreements in a spreadsheet.
Adapt to your legal needs. Choose between a lightweight default consent experience or a fully customized PDF-based form with additional fields, transfer settings, and placement controls.
Support recurring research. Consent transfer settings let you carry a participant's signature forward for 90 days or a year, so frequent participants are not asked to re-sign unnecessarily.
Common Use Cases
Use Case | Recommended Approach |
Quick studies with minimal legal requirements | Standard GQ consent checkboxes |
Organization-specific NDAs or legal agreements | Custom PDF consent form |
Recurring research programs or continuous interviews | Custom consent form with 90-day or 1-year transfer |
Multi-team organizations with different legal requirements | Team-scoped custom consent forms |
Studies requiring identity verification (name, email, address) | Custom consent form with additional required fields |
Standard GQ Consent Checkboxes
Every study includes a default consent experience out of the box. No setup is required. When participants join a study using standard consent, they see the following checkboxes inline as part of the participation flow:
- "I understand that my data is stored for research purposes for [Your company name]" (required)
- "I understand that I can request for my research participation data to be deleted at any time" (required)
- "I opt-in to being contacted for future studies" (optional)
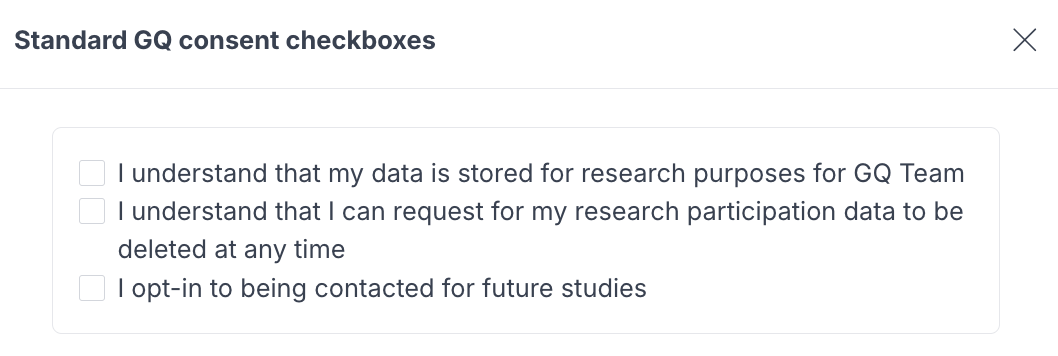
Participants must check the two required boxes to proceed. The optional opt-in checkbox controls whether participants consent to being contacted for future studies. Note that this checkbox does not appear during panel sign-up, since joining a panel implicitly opts participants in for future research. Once a participant has opted in, they will not see the checkbox again.
Custom Consent Forms
If your organization has specific legal requirements and you are on the Enterprise plan, you can upload custom consent forms as PDF documents. Custom consent forms let you present participants with your own legal language and collect additional identifying information when they sign.
Uploading a Consent Form
- Navigate to Settings > Legal.
- Under the Consent forms tab, click Attach file.
- Upload a PDF file. Any file size is supported.
- Once uploaded, your consent form appears in the list with a thumbnail preview and the upload date.
Admins can also upload a new consent form directly from a study's Plan page when selecting a consent form.
Setting a Default Form
Click Set as default next to any consent form to make it the default selection for all new studies. The default form is indicated with a "Default" badge. Only one consent form can be the default at a time.
If your organization uses teams, be aware that a default consent form can also be configured at the team level. When you assign a study to a team that has its own default consent form, that form will be automatically attached to the study. You can update or remove a team's default form under Settings > Teams > [Team Name] > Legal.
Configuring Form Fields
Click the fields button on a consent form to choose which fields participants must complete when signing.
Field | Description | Required? |
Name | Participant's full name | Optional |
Participant's email address | Optional | |
Title | Participant's job title | Optional |
Address | Participant's physical address | Optional |
Phone number | Participant's phone number | Optional |
Signature | Electronic signature | Always required |
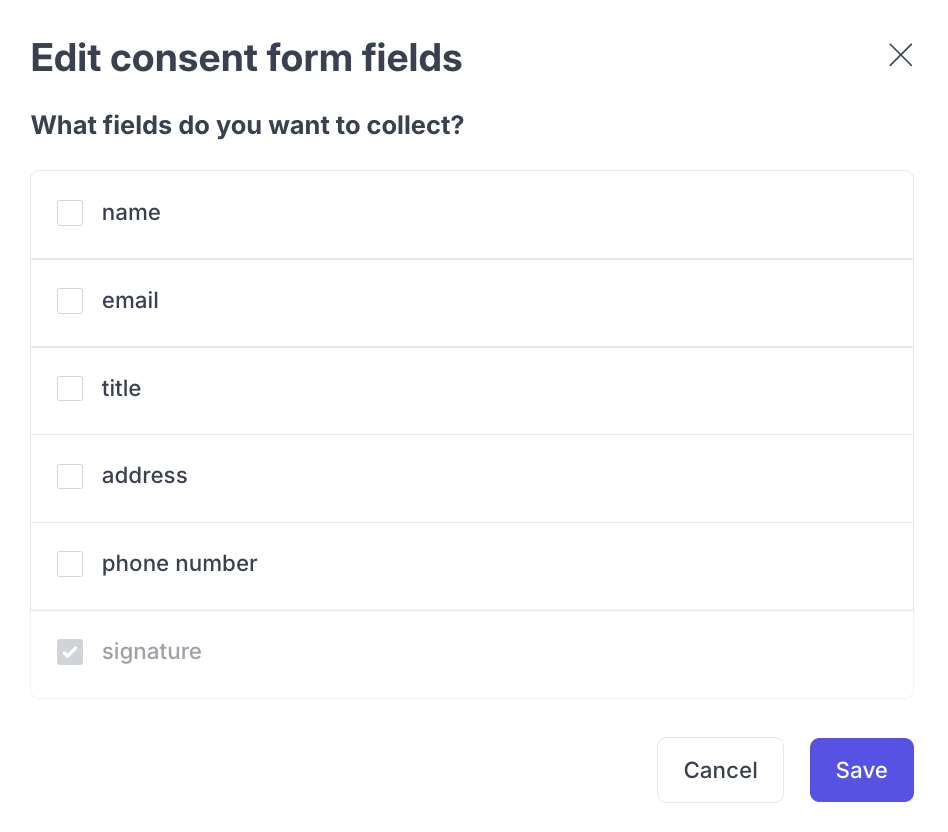
The Signature field is always required and cannot be disabled. All other fields can be toggled on or off depending on your organization's requirements.
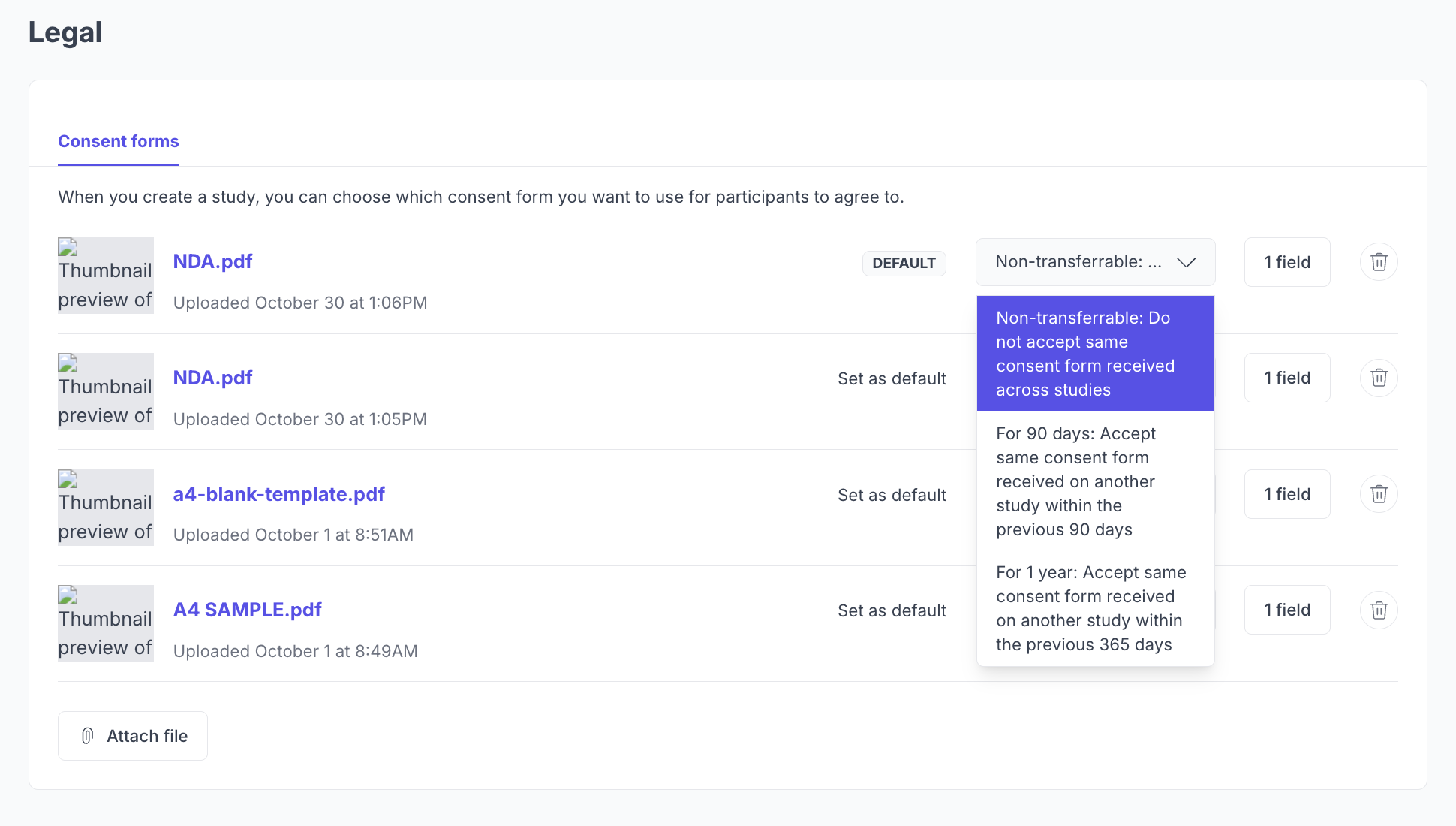
Consent Transfer Settings
Consent transfer settings control whether participants need to sign the same consent form again for each study, or if a previous signature can carry over.
Setting | Behavior |
Non-transferrable | Participants must sign the consent form for every study, even if they have signed the same form before. |
For 90 days | If a participant signed the same consent form on another study within the last 90 days, that signature is automatically accepted. |
For 1 year | If a participant signed the same consent form on another study within the last 365 days, that signature is automatically accepted. |
To configure this, go to Settings > Legal and select your preferred option under Consent Transfer Settings.
Consent Placement
If your account has consent placement feature enabled, you can control when participants see the consent form during the study flow:
- Before Screener — Participants must agree to the consent form before completing the screener survey.
- Before Participating/Booking — Participants see the consent form after the screener but before booking or participating.
To configure this, go to Settings > Legal and use the Consent Placement dropdown.
Team-Scoped Consent Forms
If your organization uses teams, consent forms can be scoped to specific teams:
Unscoped forms are available to all teams and appear in every study's consent dropdown. Team-scoped forms are only available to studies belonging to that team. To upload a team-scoped consent form, navigate to Settings > Teams > [Team Name] > Legal and upload the form from there.
When selecting a consent form for a study, only forms that are unscoped or scoped to the study's team will appear in the dropdown.
Deleting a Consent Form
Click the delete button next to a consent form to remove it. Deleting a consent form also removes it from any studies it was attached to. Previously signed copies are not affected.
Using Consent Forms in Studies
Selecting a Consent Form
- Open your study and navigate to the Plan page.
- In the Consent section, use the dropdown to choose a consent form:
- Standard GQ consent checkboxes — the built-in checkboxes (default).
- [Your uploaded form name] — a custom PDF consent form you have uploaded.
- Upload new... (admin only) — upload a new PDF directly from the study.
- Click the Preview button (eye icon) to see how the consent form will appear to participants.
If your account has a default consent form set, it will be pre-selected for new studies. When duplicating a study, the attached consent form is copied to the new study automatically.
The Participant Experience
Standard Consent
When a study uses standard GQ consent checkboxes, participants see the consent checkboxes inline as part of the participation flow. They must check the required boxes before they can proceed.
Custom Consent Form
When a study uses a custom PDF consent form, participants go through the following steps:
- View the consent form. The PDF is displayed in a full-width viewer. Participants can scroll through and read the entire document.
- Sign the consent form. A fixed bar at the bottom of the page prompts them to sign. Clicking the sign button opens a modal where they fill in the required fields (name, email, signature, etc.) as configured by the researcher.
- Submit. After completing all required fields, the participant submits the form and is redirected back to the study participation flow.
A signed PDF is automatically generated that includes a front page with metadata (study name, participant details, signature, signing date, and IP address) followed by the original consent form PDF.
The consent form signing experience supports multiple languages, including English, German, Spanish, French, Italian, Portuguese (Brazil), and Turkish.
Viewing Signed Consent Forms
From a Participant Profile
Signed consent forms can be viewed from several locations:
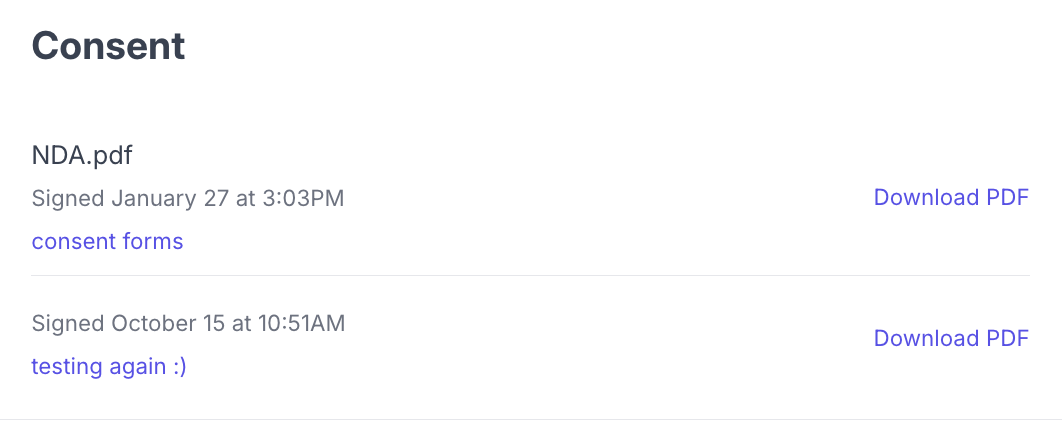
Participant profile slideout — Open a participant's profile in any study to see their signed consent forms in the Consent section. Candidate profile — Navigate to a candidate's profile in your panel to see all consent forms they have signed across studies.
Each signed consent form displays the form filename, the date and time it was signed, the study it was signed for (with a link to the study), and a Download PDF link to download the signed copy.
Bulk Export
You can export signed consent forms in bulk from a study's participant list. Select the participants you want to export consent forms for, then use the bulk actions menu to export. A download link will be sent to your email.
Best Practices
Set a default consent form early. If your organization requires a custom consent form for most studies, set it as the default in Settings > Legal so it is automatically attached to every new study. This prevents studies from going live without the right consent form.
Use transfer settings for recurring research. If you run continuous discovery or regular interview programs, set the consent transfer to 90 days or 1 year to reduce friction for returning participants.
Test on both desktop and mobile. Before launching a study, preview the consent form on a mobile device to confirm it renders correctly. Some embedded PDF signature fields may not display properly on older mobile browsers.
Review team-level defaults when creating cross-team studies. If your teams have different default consent forms, double-check which form is attached when you assign a study to a team.
Audit signed forms periodically. Use the bulk export feature to download signed consent forms for compliance audits or legal reviews, rather than checking profiles one by one.
Troubleshooting
Display and Rendering Issues
Issue | What's Happening | How to Fix It |
Consent form not appearing for participants | The consent form was not attached to the study, or the study is using standard checkboxes instead of a custom form. | Open the study, go to Plan > Consent, and confirm the correct consent form is selected. If the form is missing from the dropdown, verify it is either unscoped or scoped to the study's team. |
Consent form footer overlaps content | On some screen resolutions, the fixed signing bar at the bottom of the viewer overlaps the last portion of the PDF. | Participants can scroll to reveal the hidden content. Advise participants to scroll past the footer before signing. |
Participants on mobile cannot complete the signature | The embedded PDF signature field may not render properly on certain mobile browsers. | Advise participants to open the consent form on a desktop browser, or update their mobile browser to the latest version. |
Upload and Setup Issues
Issue | What's Happening | How to Fix It |
Cannot upload a consent form | The file type is not supported, or the user does not have Admin permissions. | Confirm the file is a PDF and that you are signed in as an Admin. If the Attach file button is not visible under Settings > Legal, check your role settings. |
Consent form not copied when duplicating a study | The attached consent form was not carried over to the new study. | Re-attach the consent form manually from the duplicated study's Plan > Consent dropdown. This was a known bug and has been fixed for future duplications. |
Unrecognized consent form automatically attached to a study | The study was assigned to a team that has its own default consent form configured under Settings > Teams > [Team Name] > Legal. | Check the team's legal settings to update or remove the team-level default. |
Consent form not applied to new studies | No default consent form is set. | In Settings > Legal, click Set as default after uploading your preferred consent form. |
Signing and Submission Issues
Issue | What's Happening | How to Fix It |
Participants asked to re-sign a form they already signed | The consent transfer setting is set to Non-transferrable, which requires a new signature for every study. | Go to Settings > Legal > Consent Transfer Settings and choose For 90 days or For 1 year so previously signed forms carry over. |
"No consent" flag on manually imported candidates | Imported candidates have not opted in through the platform, and the account's eligibility rules require an explicit opt-in. | Adjust the eligibility rule (e.g., set the opt-in condition to "is not true") or exclude imported candidates from the rule. |
Signed Form Access Issues
Issue | What's Happening | How to Fix It |
Signed consent form not showing in participant profile | The participant exited before completing all required fields and submitting, or the form was signed by an external recruit before a display fix was deployed. | Check whether the participant completed the signing process. If incomplete, resend the study link. For external recruit issues, contact support. |
Cannot download signed consent form PDF | Browser pop-ups are blocked, or the download link is not rendering. | Allow pop-ups for Great Question in your browser, then go to the participant's profile > Consent section > Download PDF. If the link is missing entirely, contact support. |
FAQ
Question | Answer |
Can I use different consent forms for different studies? | Yes. Each study can have its own consent form selected independently. You can also set a default form so new studies start with your preferred option. |
What file types are supported for custom consent forms? | Only PDF files are supported. There is no file size limit. |
What happens if I delete a consent form that is attached to a study? | The consent form will be removed from the study, and the study will revert to using no custom consent form. Previously signed copies are not affected. |
Is the signature field required? | Yes. The signature field is always required on custom consent forms and cannot be disabled. |
Why don't participants see the "opt-in for future studies" checkbox during panel sign-up? | Joining a panel implicitly opts participants in for future research, so the checkbox is not shown. It only appears for non-panel studies. Once a participant has opted in, they will not see the checkbox again. |
Still need help? Contact us at [email protected] — we're happy to help!
